Abstract
Introduction
Hemophilia B is an X-linked inherited bleeding disorder which is caused by mutations in the F9 gene. Hemophilia B Leyden is a rare subtype of hemophilia B, which is caused by mutations within a 40-basepair region in the F9 promoter. Hemophilia B Leyden account for 1.9% of hemophilia B, Hemophilia B Leyden is characterized by low factor IX activity (FIX:C) during childhood and gradual increase in puberty. To date, more than 100 cases of hemophilia B Leyden and 21 distinct point mutations have been reported. However, the genotype-phenotype correlations have not yet been established. Nearly all reported hemophilia B Leyden cases are Caucasian. The first case of Japanese patient with Heophilia B was reported recently (Yamashita et al. Int J Hematol. 2017). There have been only two other reported cases of hemophilia B Leyden in Asia, one from Thailand (c. -19 T>C), the other from China (c. -21 T>A). Here, we report the distinct Japanese brother cases with hemophilia B Leyden. DNA sequencing analysis revealed HGVS (c. -35 G>A) ONECUT binding site mutation, which was the same as the first reported Japanese hemophilia B Leyden case. All three Japanese hemophilia B Leyden patients improved during early childhood.
Methods
Informed consent was obtained from guardians. The study was approved by the Ethics Committee on Medical Research of Tokyo Medical University. DNA sequences were analyzed by direct sequencing.
Cases
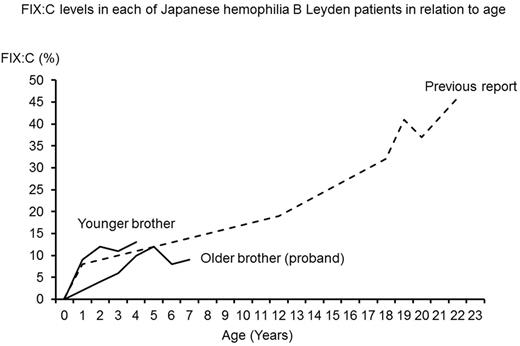
A previously healthy six months old boy developed subcutaneous hematoma after vaccination. There was no family history of hemophilia. He was diagnosed as hemophilia B based on the results of APTT 54sec and FIX:C <1%. FIX:C increased with 4% at 1 year of age, 6% at 3 years of age and 9% at 7 years of age [Figure]. He has been free from major bleeding events.
Younger brother of the above-mentioned proband was born at 39 weeks gestation with birth weight of 2988g. Although he had no symptoms, he underwent an examination at 1 month of age because of his family history. He was found to have hemophilia B with APTT 58sec and FIX:C <1%. FIX:C increased with 9% at 1 year of age and 13% at 4 years of age. He had bleeding episode of traumatic lip injury at 1 year of age.
We identified a ONECUT binding site mutation (c.-35G>A) in the promoter region of FIX gene (F9) in both patients. Both of their parents were Japanese. This mutation was the same as that identified in the first Japanese case.
Discussion
Here, we report Japanese brother cases with hemophilia B Leyden. Hemophilia B Leyden is caused by point mutation of proximal promoter region, which includes binding sites for three transcription factors, hepatic nuclear factor 4α (HNF4α), CCAAT enhancer-binding protein α (C/EBPα), and ONECUT1/2. Mutations in these transcription factor binding sites are postulated to disrupt transcriptional activation of F9 . Androgen receptor (AR) is also known to activates F9 transcription in the presence of androgen. It is thought that increased androgen secretion after puberty induce binding of AR to androgen receptor element (ARE) and activates transcription of F9 . In our brother cases with hemophilia B Leyden, FIX:C rose during early childhood, which was different from other hemophilia B Leyden, in which FIX:C rise in adolescence. It was thought that these phenomena might be associated with binding of ONECUT transcription factor to this site. Previously reported another Japanese hemophilia B Leyden patient also showed similar increase in FIX:C level during early childhood.
Conclusion
Our brother cases and previous report suggested that c. -35 G>A ONECUT binding site mutation might be associated with earlier rise of FIX:C level than other transcription factor binding sites mutation. We should be aware that hemophilia B Leyden is seen not only in Caucasian but also in Asian including Japanese.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal